- Email : [email protected]
- Address: Toronto Canada
- Delivery: Free Delivery From $100CAD
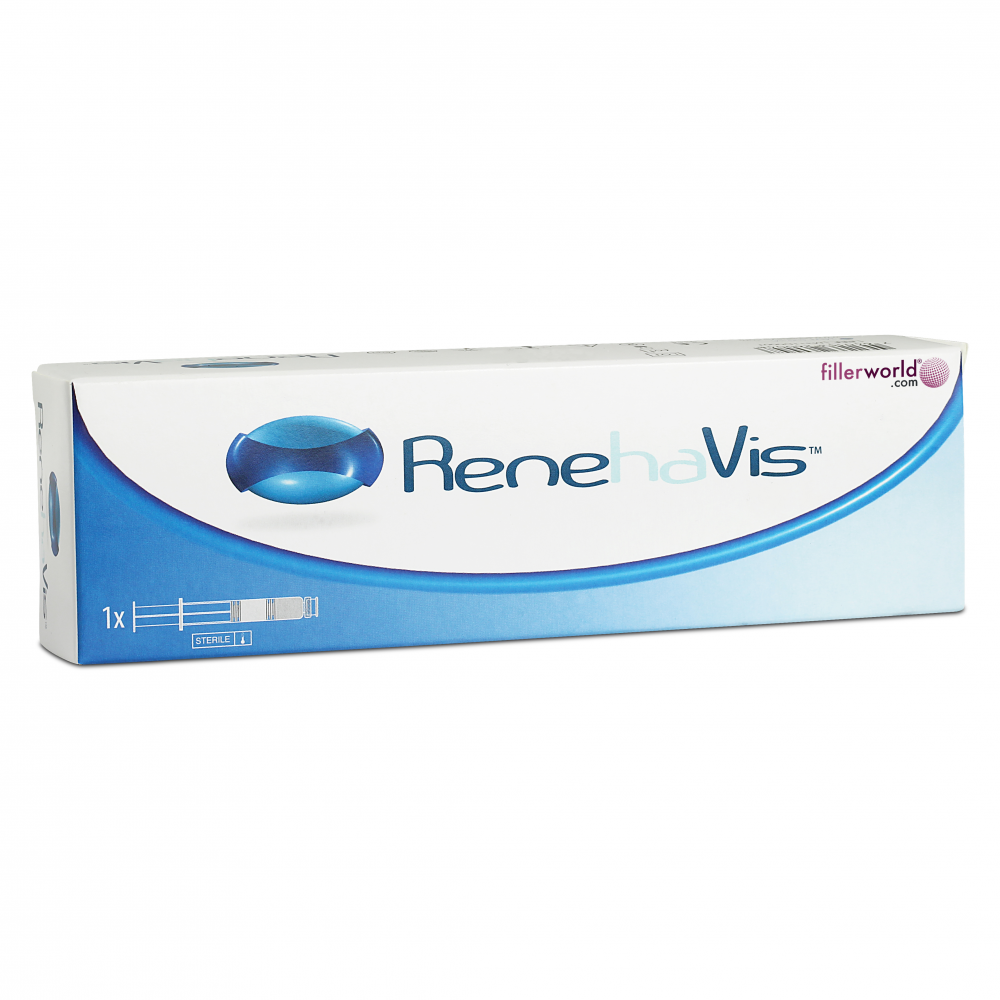
Buy RenehaVis Online
CAD $82.00
One pre-filled syringe of 15.4mg/0.7ml
Description
Buy RenehaVis Online
Buy RenehaVis Online
What is RenehaVis?
RenehaVis is a sodium hyaluronate product, comprising a dual-chambered prefilled syringe that enables administration of both high and low molecular weight hyaluronate in a single intra-articular injection. Using high and low molecular weight sodium hyaluronate improves both cushioning and elasticity, enabling improvement in movement and pain relief at rest and during activity.
A product designed to relieve pain and stiffness of the knee joint in patients with degenerative changes to the synovial joint, including osteoarthritis. RenehaVis contains 2 different Hyaluronic Acids with 2 different concentrations in 1 syringe, either 15.4mg/0.7ml or 7.0mg/0.7ml.
Instruction for use
Presentation
RenehaVis™ is two clear solutions of sterile sodium hyaluronate in a phosphate buffered saline contained in a two chamber prefilled syringe for single intra-articular injection into the synovial space of the joint.
RenehaVis™ LMW Sodium hyaluronate 15.4mg/0.7ml and HMW Sodium hyaluronate 7.0mg/0.7ml For the relief of the symptoms of osteoarthritis of the knee by providing support and lubrication to the knee joint.
RenehaVis™ 0.7ml LMW and 0.7ml HMW, terminally sterilised by moist heat, is enclosed within a glass, ready to use, disposable syringe. The syringe is packed within a blister pack and an outer cardboard carton.
RenehaVis™ is a sterile pre-filled two-chamber glass ready to use disposable syringe containing:
Buy RenehaVis Online
- Chamber 1 >
Sodium hyaluronate Low Molecular Weight
(LMW): 0.7ml sterile 2.2% sodium hyaluronate
1×106 Da molecular weight. - Chamber 2 >
Sodium hyaluronate High Molecular Weight
(HMW): 0.7ml sterile 1.0% sodium
Dosage and Administration
Injection of RenehaVis™ should only be made by a Healthcare Professional trained in the technique.
The dosage regimen is injection into the affected synovial joint space once a week for up to three injections depending on the severity of the degenerative change to the knee joint.
Clean the skin around the injection site with antiseptic and allow to dry before injection is given. If joint effusion is present it should be aspirated before injection of RenehaVis™.
Aspiration of a small amount of synovial fluid as part of the injection procedure to ensure the correct positioning of the needle is possible. Before proceeding, ensure that the plunger rod is tightly screwed into the plunger stopper.
The contents of the syringe are sterile and should be injected using a sterile needle of an appropriate size (25 gauge needle is recommended). The syringe is fitted with a Luer lock (6%). Discard the syringe and needle after single use.
Uses
For the relief of pain and stiffness of the knee joint in patients with degenerative changes to the synovial joint. The duration of effect in patients with grade 1 to 3 medial compartment osteoarthritis has been demonstrated to be up to twelve months.
The performance of RenehaVis™ is due to its biocompatibility and physicochemical properties. The LMW and HMW sodium hyaluronate contained in RenehaVis™ is a biopolymer composed of repeating disaccharide units of N-acetylglucosamine and glucuronic acid and though it is biosynthesised by the bacterium Streptococcus equi it has been shown to be the same as the sodium hyaluronate which is found in the human body. RenehaVisTM supplements the endogenous Sodium Hyaluronate found naturally in the synovium but which has been depleted by degenerative and traumatic changes to the synovial joint.
Contra-indications
Patients with known sensitivity to sodium hyaluronate.
Warnings and Precautions
Do not inject RenehaVis™ if the area of the injection is infected or where there is evidence of skin disease. RenehaVis™ pre-filled syringe is single use. The contents of the syringe should be used for one injection only. Any remaining sodium hyaluronate should be discarded. If a syringe is retained for a subsequent injection there is a risk of contamination resulting in the possible infection of the patient and/or foreign body reaction. RenehaVis™ should not be resterilised as the device performance may be compromised which could cause serious harm to the patient’s health and safety. Sodium hyaluronate is manufactured by fermentation of Streptococcus equi and rigorously purified. However, the physician should consider the immunological and potential risks that can be associated with the injection of any biological material.
Do not use for children. There is no evidence concerning the safety of RenehaVis™ in human pregnancy and lactation. Administration during pregnancy and lactation is at the discretion of the orthopaedic surgeon. Do not use if sterile packaging has been damaged. Do not use after the expiry date. Follow national or local guidelines for the
safe use and disposal of needles. Obtain prompt medical attention if injury occurs.